. The chemistry of plant and animal life. Agricultural chemistry. NITRIC ACID AND NITROGEN COMPOUNDS 83 the cylinder. The delivery tube should pass into and nearly to the bottom of a test tube which is immersed in cold water in the cylin- der. Put 15 cc. concentrated sulfuric acid (H2SO4) and 10 grams of either sodium nitrate (NaN03) or potassium nitrate (KN03) into the flask and apply heat until about 4 or 5 cc. of HN03 are distilled and collected in the test tube. Do not remove the flame unless the end of the delivery tube is above the liquid in the test tube, otherwise the liquid will be dr
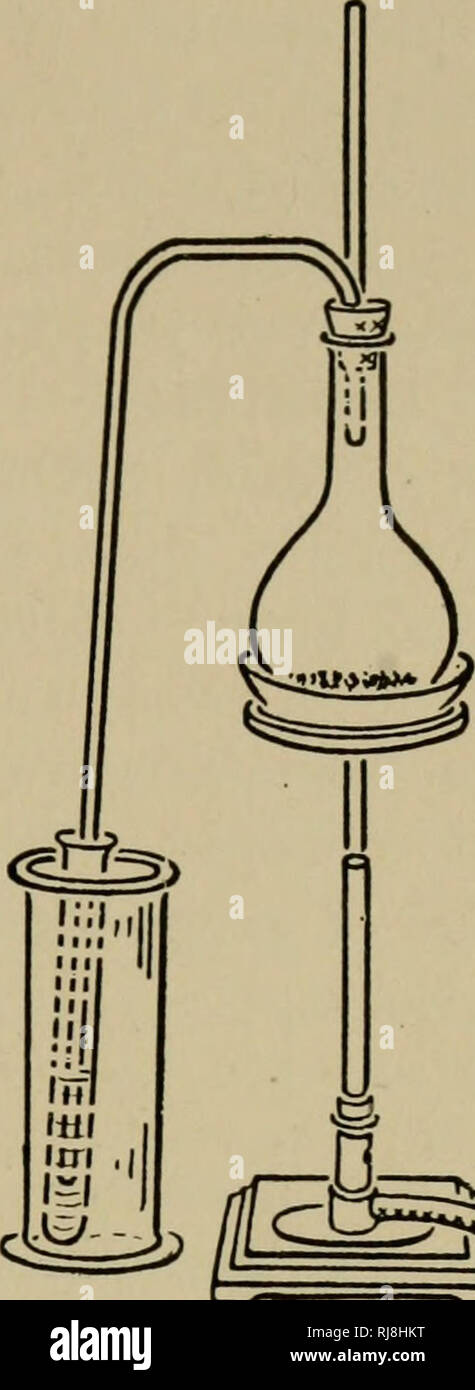
Image details
Contributor:
Library Book Collection / Alamy Stock PhotoImage ID:
RJ8HKTFile size:
7.1 MB (192.1 KB Compressed download)Releases:
Model - no | Property - noDo I need a release?Dimensions:
956 x 2612 px | 8.1 x 22.1 cm | 3.2 x 8.7 inches | 300dpiMore information:
This image is a public domain image, which means either that copyright has expired in the image or the copyright holder has waived their copyright. Alamy charges you a fee for access to the high resolution copy of the image.
This image could have imperfections as it’s either historical or reportage.
. The chemistry of plant and animal life. Agricultural chemistry. NITRIC ACID AND NITROGEN COMPOUNDS 83 the cylinder. The delivery tube should pass into and nearly to the bottom of a test tube which is immersed in cold water in the cylin- der. Put 15 cc. concentrated sulfuric acid (H2SO4) and 10 grams of either sodium nitrate (NaN03) or potassium nitrate (KN03) into the flask and apply heat until about 4 or 5 cc. of HN03 are distilled and collected in the test tube. Do not remove the flame unless the end of the delivery tube is above the liquid in the test tube, otherwise the liquid will be drawn back into the flask. Make the following tests with HN03. (1) Remove a drop of the acid by means of a glass tube, and apply it to a piece of either woolen cloth or silk. Observe the result. (2) Place a few drops of indigo solution in a test tube containing 5 cc. of water, then add about 2 cc. of HNO3. Observe the result. (3) Place a small piece of copper in the test tube containing the remainder of the acid. Observe the result. If no reaction takes place, add a little water. Do not pour the contents of the flask into the sink or waste jars until cool, otherwise the hot acid coming in contact with cold water may cause spattering of the acid. Questions.— (1) Why was H2S04 used in the preparation of this compound ? (2) What material supplied the N03 radical ? (3) Write the reaction which took place in the flask after heat was applied. (4) Is HNO3 a solid, liquid, or gas ? What is the proof ? (5) What caused the red fumes to be given off when the copper was added to the test tube ? (6) Does HN03 give off H when a metal is added to it ? Why ? (7) Why did the HN03 bleach the indigo solution ? (8) Why is ordinary HN03 colored yellow ? (9) Is HN03 an active or inert chemical ? (10) What is a nitrate ?. a* Fig. 42. — Preparation of nitric add. 94. Properties. — When pure, nitric acid is a colorless liquid; the commercial acid has a yellow color because. Please note that these i