. Plant physiology. Plant physiology. ASSIMILATION OF CARBON 23 light screens and the latter the prismatic spectrum. Both came to the con- clusion that plants decompose carbon dioxide most readily under the influence of the yellow light rays. Sachs^ divided the spectrum into two nearly equal portions, by using a solution of potassium dichromate and one of ammoniacal copper oxide, and found that decomposition of carbon dioxide proceeded almost as energetically in the yellow portion of the spectrum as in direct sunlight, while very little decomposition occurred in the blue-violet region. It is s
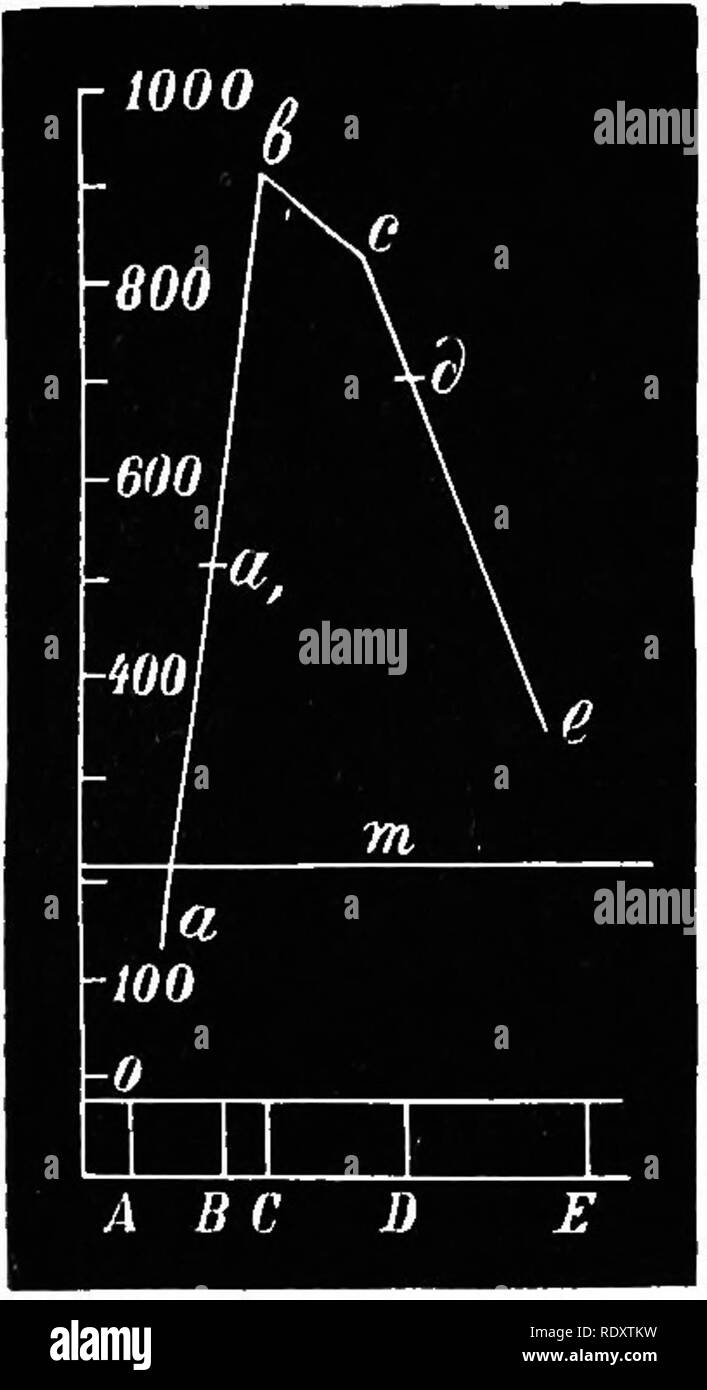
Image details
Contributor:
The Book Worm / Alamy Stock PhotoImage ID:
RDXTKWFile size:
7.1 MB (141.8 KB Compressed download)Releases:
Model - no | Property - noDo I need a release?Dimensions:
1166 x 2143 px | 19.7 x 36.3 cm | 7.8 x 14.3 inches | 150dpiMore information:
This image is a public domain image, which means either that copyright has expired in the image or the copyright holder has waived their copyright. Alamy charges you a fee for access to the high resolution copy of the image.
This image could have imperfections as it’s either historical or reportage.
. Plant physiology. Plant physiology. ASSIMILATION OF CARBON 23 light screens and the latter the prismatic spectrum. Both came to the con- clusion that plants decompose carbon dioxide most readily under the influence of the yellow light rays. Sachs^ divided the spectrum into two nearly equal portions, by using a solution of potassium dichromate and one of ammoniacal copper oxide, and found that decomposition of carbon dioxide proceeded almost as energetically in the yellow portion of the spectrum as in direct sunlight, while very little decomposition occurred in the blue-violet region. It is seen, therefore, that it is not the so-called "chemical" rays that are needed for this process, but chiefly the less refrangible rays of the first half of the spectrum. Sachs determined the amount of oxygen given off, using the method of counting gas bubbles (Fig. 2). C, v The next problem was to discover in what rays of the sfirt half of the spectrum the decomposition of carbonic acid was most rapid. The most exact studies upon this point were carried out by Timiriazev^ who arranged his experi- ments as follows: Sunlight was reflected from a helio- stat into a dark chamber and was then broken up by a carbon bisulphide prism. Pieces of bamboo leaves were enclosed in glass tubes, with air containing 5 per cent, of carbon dioxide, and these tubes were placed in various regions of the spectrum—in the red between A and B, in the chlorophyll absorption band between B and C, in the orange, in the yellow, and in the green. At the conclusion of the experiment analyses of the gas were made, by means of a very sensitive appa- ratus capable of measuring extremely small amounts of gas. Timiriazev's results are graphically repre- sented in Fig. 13. The ends of the five ordinates, for the five positions in the spectrum where the tubes were exposed, are joined to form a curve, which represents the relative rates of decomposition of carbon dioxide in these different regions of the