Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . Fig. 89.—Elements of Carbon Cylinder Cell with Depolarizer.Zinc, manganese dioxide in a porous cup and sal-ammoniac solution. manganese and then sealed in. The zinc rod is prevented fromtouching the carbon by being first inserted through a porce-lain insulator. About 4 to 6 ounces of sal-ammoniac are gen-erally used for cells of ordinary size. The salt is placed inthe jar, water poured in until it is abouttwo-thirds full, and then stirred till all thesalt is dissolved. When the carbo
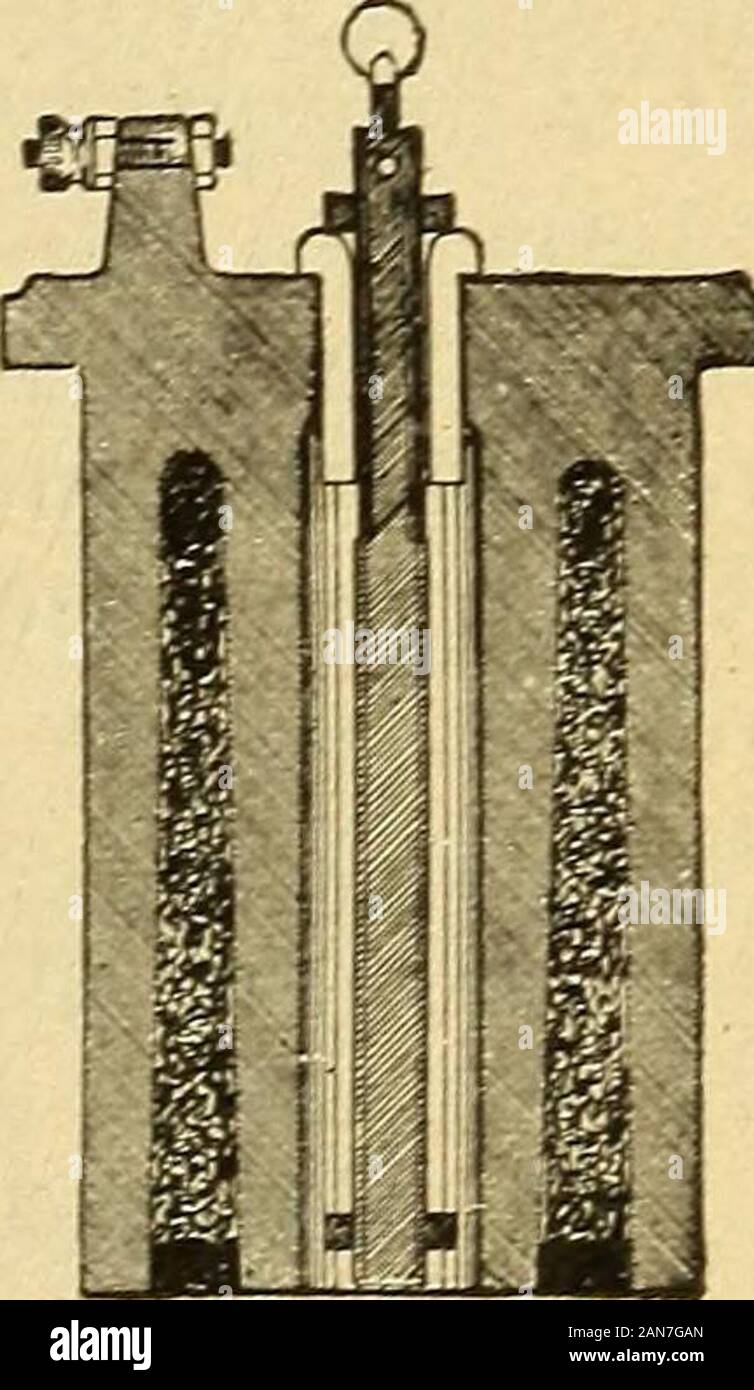
Image details
Contributor:
The Reading Room / Alamy Stock PhotoImage ID:
2AN7GANFile size:
7.1 MB (276.7 KB Compressed download)Releases:
Model - no | Property - noDo I need a release?Dimensions:
1204 x 2074 px | 20.4 x 35.1 cm | 8 x 13.8 inches | 150dpiMore information:
This image is a public domain image, which means either that copyright has expired in the image or the copyright holder has waived their copyright. Alamy charges you a fee for access to the high resolution copy of the image.
This image could have imperfections as it’s either historical or reportage.
Lessons in practical electricity; principles, experiments, and arithmetical problems, an elementary text-book . Fig. 89.—Elements of Carbon Cylinder Cell with Depolarizer.Zinc, manganese dioxide in a porous cup and sal-ammoniac solution. manganese and then sealed in. The zinc rod is prevented fromtouching the carbon by being first inserted through a porce-lain insulator. About 4 to 6 ounces of sal-ammoniac are gen-erally used for cells of ordinary size. The salt is placed inthe jar, water poured in until it is abouttwo-thirds full, and then stirred till all thesalt is dissolved. When the carbon cylinderis inserted the solution should be wTithin inches of the top of the jar. These cellsshould not be put in wrarm places, as overthe heater in a cellar, on account of therapid evaporation of the electrolvte. TheE. M. F. is from 1.4 to 1.6 volts for thedifferent forms of this type. 91. Edison-Lalande Cell.—As in theLeclanche type, this cell is a single fluidbattery with solid depolarizer, but is ad-. Fig. 90. Section through car- mirably adapted for use—im closed circuit bnn cylinder showing i p -ii i • MnOo depolarizer. work, as tor small motors, electrotypmg, telegraphy, etc. Zinc is the positive, and black oxide ofcopper (CuO), the negative element. The exciting liquidis a solution of caustic potash. The oxide of copper is ob-tained by the process of roasting copper turnings ; it is then 74 PRACTICAL ELECTRICITY. ground into a fine powder and compressed into solid blocks, from which plates of a suitable size are cut. These plates are suspended from the cover of the battery jar, Fig. 91, by a light framework of copper, one end of the frame-work terminating as the positive pole of the battery. On each side of the copper oxideelement is suspended a zincplate, which is prevented fromcoming into contact with thecopper oxide plate by means ofvulcanite buttons. When thecircuit is closed and the cell inaction, the water is decom-posed, the ox}^gen, formingwith the zinc,