Potassium electron configuration. Illustration of the atomic structure and electron configuration of the element potassium. An atom of this element ha
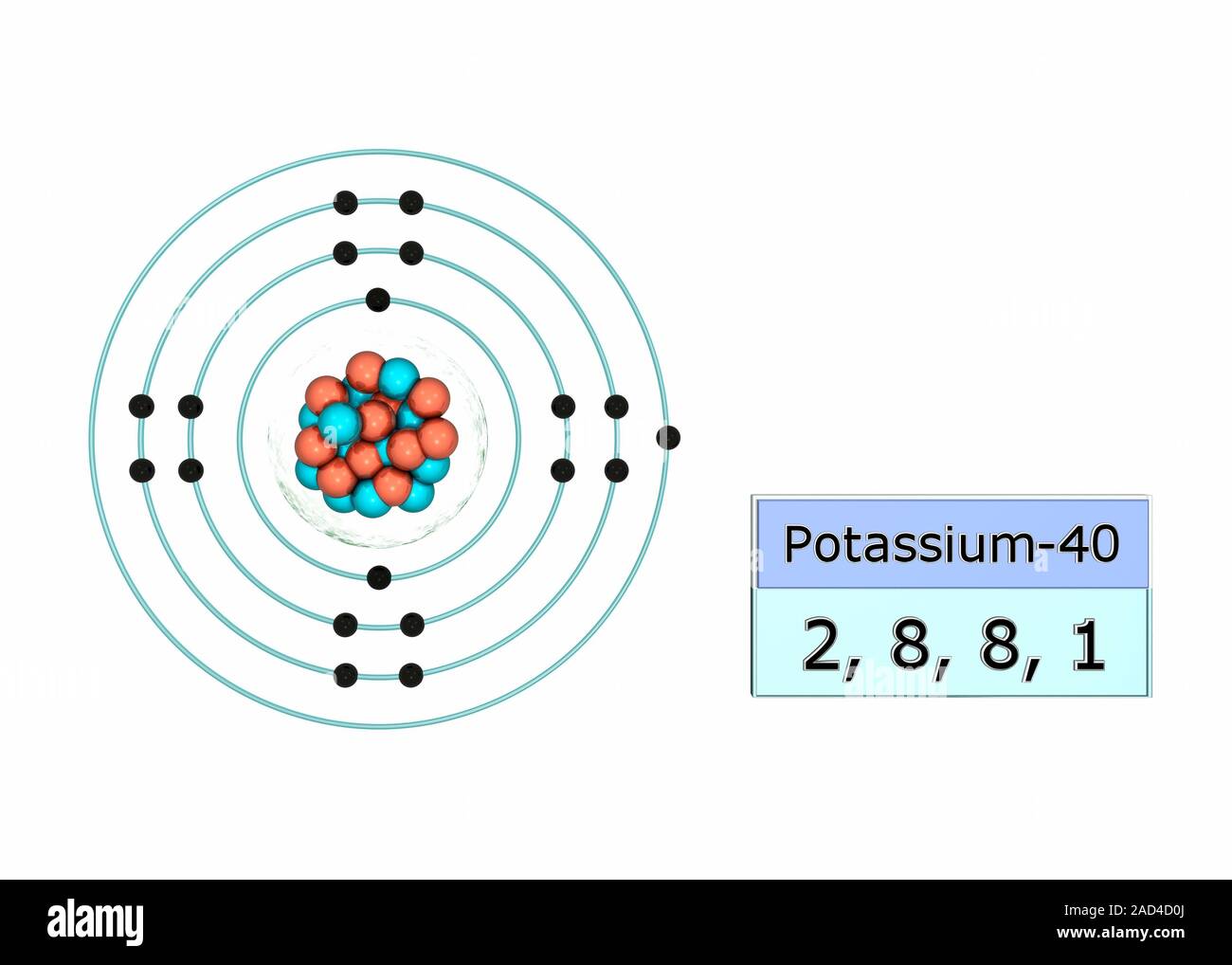
Image details
Contributor:
Science Photo Library / Alamy Stock PhotoImage ID:
2AD4D0JFile size:
50.3 MB (472.3 KB Compressed download)Releases:
Model - no | Property - noDo I need a release?Dimensions:
4961 x 3543 px | 42 x 30 cm | 16.5 x 11.8 inches | 300dpiDate taken:
28 April 2016Photographer:
ANIMATE4.COM/SCIENCE PHOTO LIBARYMore information:
Potassium electron configuration. Illustration of the atomic structure and electron configuration of the element potassium. An atom of this element has 19 electrons (black dots) in four shells around the nucleus. There are two electrons in the first shell (both in the 1s orbital), and eight electrons each filling both the second shell (the 2s and 2p orbitals) and the third shell (the 3s and 3p orbitals). Finally, there is one electron in the fourth shell (the 4s orbital). The electron configuration is 2, 8, 8, 1. The isotope shown here is potassium-40, with a nucleus of 21 protons (red) and 19 neutrons (blue). All isotopes of an element have the same electronic structure.