. The Family tutor . stances;while sulphuric acid, which has threeequivalents of base to one of acid, is notan electrolyte. No two elements seemcapable of forming more than one elec-trolyte. 6. A single ion, as bromine, for in-stance, has no disposition to pass to eitherof the electrodes, and the current has noeffect upon it. There can be no electro-lysis except when a separation of ionstakes place, and the separated elementsgo one to each electrode. 7. There isno such thing, in fact, (as has been oftensupposed), as an actual transfer of ionsfrom one part of the fluid to either elec-trode. In
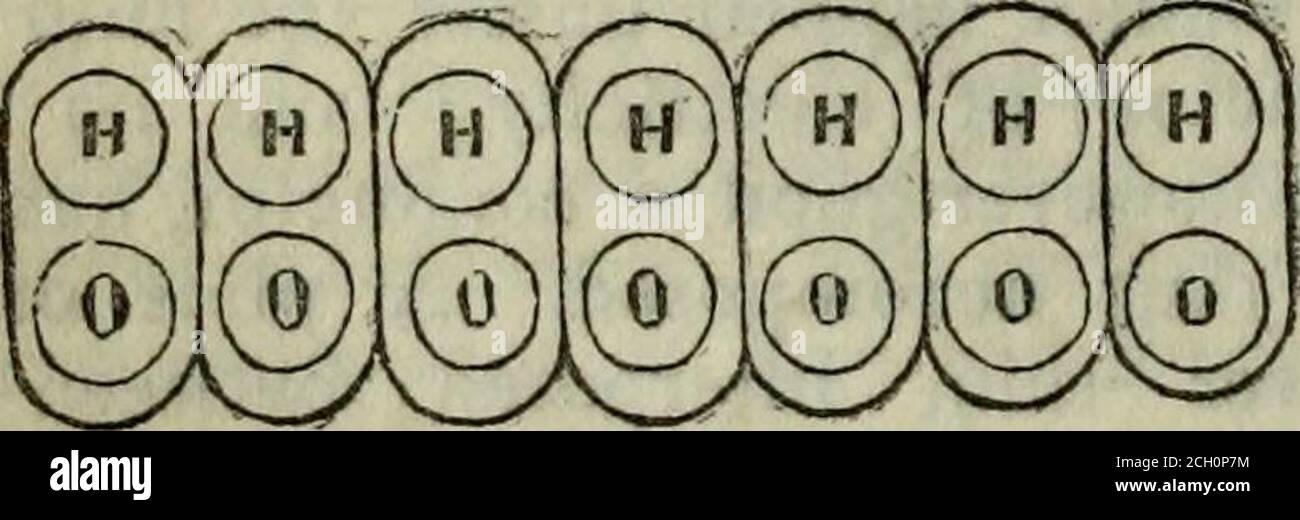
Image details
Contributor:
Reading Room 2020 / Alamy Stock PhotoImage ID:
2CH0P7MFile size:
7.1 MB (231.4 KB Compressed download)Releases:
Model - no | Property - noDo I need a release?Dimensions:
2745 x 910 px | 23.2 x 7.7 cm | 9.2 x 3 inches | 300dpiMore information:
This image is a public domain image, which means either that copyright has expired in the image or the copyright holder has waived their copyright. Alamy charges you a fee for access to the high resolution copy of the image.
This image could have imperfections as it’s either historical or reportage.
. The Family tutor . stances;while sulphuric acid, which has threeequivalents of base to one of acid, is notan electrolyte. No two elements seemcapable of forming more than one elec-trolyte. 6. A single ion, as bromine, for in-stance, has no disposition to pass to eitherof the electrodes, and the current has noeffect upon it. There can be no electro-lysis except when a separation of ionstakes place, and the separated elementsgo one to each electrode. 7. There isno such thing, in fact, (as has been oftensupposed), as an actual transfer of ionsfrom one part of the fluid to either elec-trode. In the case of water, for example, oxygen is given out on one side andhydrogen on the other. In order thatthis may be the case, there must be waterbetvs^een the electrodes. We cannot be-lieve that the separation of the elementstakes place at the electrode where oneelement is evolved, and the other travelsover unseen to the opposite electrode.We may, however, conceive of water in itsquiet state, as represented by Fig. 44, . cules of water undergohig electrolysis, the H and O being eliminated at the Fig. 44.. each molecule being firmly united bypolar attractions to every other, and thatthe electrolytic force of the electric cur-rent has power to disturb this polarequilibrium, each molecule being similarlyaffected. In this case the electrolysis willproceed from particle to particle throughthe whole chain of affinities, decomposingand recomposing, until the ultimate par-ticle on each side, having no polar force toneutralize it, escapes at that electrodewhich has a polarity opposite to itself.This explanation may be better understood, perhaps, by inspecting Fig. 45, whichrepresents a series of compound mole-